
50% of the World suffers from
Acute & Chronic Pain…
and we have the solution!
$1,235,000 Raised

50% of the World suffers from
Acute & Chronic Pain
and we have the solution!
$1,235,000 Raised

Offering Terms
$618,000
Maximum Offering
$24,000
Target Offering
$300
Minimum Investment
$3.00
Unit Price
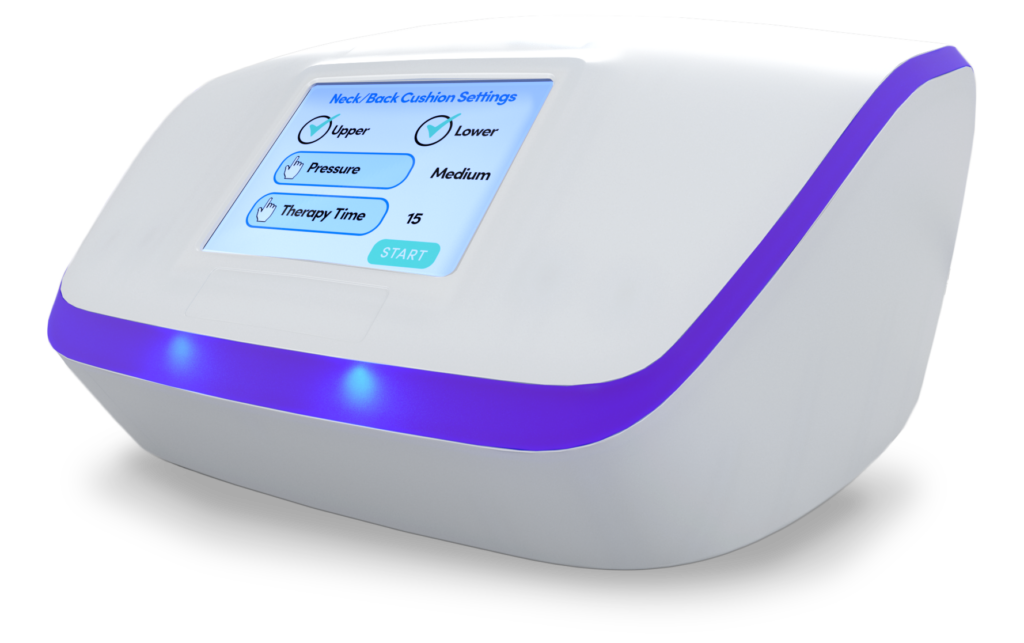
IN-CLINIC AND AT-HOME THERAPY
Eva Medtec is at the forefront of anatomical medicine, pioneering medical device solutions that address pain management and lymphatic circulation.
NEUROGLIDE’S BACK AND NECK PAD
Our flagship product, Neuroglide, automates a specialized technique that simulates manual lymphatic drainage (MLD) therapy, which is known to treat various conditions.

- Arthritis
- Back Injuries
- Sports Recovery
- Lymphedema
- Fibromyalgia
- Post-Op Edema
About Neuroglide:
IN-CLINIC AND AT-HOME USE
BACK & NECK PAD
PAIN RELIEF & RECOVERY

How The Neuroglide Back / Neck Pad Works
Neuroglide works by applying 16 precise Stretch n’ Release air bladder pressures that simulate manual lymphatic drainage (MLD) therapy.
The gentle pressures to the lymph capillaries and superficial fascia just under the skin accelerates removal of lymphatic waste while manipulating the superficial fascia so it can help improve lymph flow, and reduce the inflammation that causes pain and lymph congestion.
- Accelerate Lymphatic Contractility
- Increase Lymph Flow
- Significant Pain Reduction
- Reduce Recovery Time
- Promote Relaxation & Improve Sleep
- Transform Patient Outcomes
Rhythmic
Stretch n’ Release Air Bladders
Stretch n’ Release Air Bladders
Stimulates
Lymphatic Circulation
Manipulates
Superficial Fascia
Superficial Fascia
Removes
Waste / Toxins
Waste / Toxins
FUTURE Rx & OTC THERAPY ATTACHMENTS:
Neuroglide's Smart Valve Therapy Attachment Technology
Each attachment provides the same Stretch n’ Release air bladder technology using our patented Smart Valve technology. Neuroglide future therapy Rx and OTC attachments already passed Intertek electrical safety standards, but still need to complete development, clinical testing and obtain FDA clearance.

Knee Wrap
- Post-Op Knee Surgery Recovery
- Gout Arthritis Pain & Swelling
- Sports Injuries

Scalp Wrap
- Traumatic Brain Injury
- Migraines
- Sports Injuries

Calf-Foot Wrap
- Achilles Injuries
- Foot / Ankle Pain
- Sports Injuries

Mini Back Pad
- Low Back Pain
- Neck Pain
- Sports Injuries
Why is Neuroglide essential:
The Lymphatic System: The Body’s Hidden Highway for Health
A Healthy Lymphatic Circulatory System
The lymphatic system is a crucial part of the immune system. It acts as a drainage network, removing waste, toxins, and excess fluid from tissues while transporting immune cells to fight infection.
By supporting the natural drainage of lymphatic fluid, Neuroglide helps restore the body’s ability to detoxify, reduce pain, and promote healing, ensuring a more effcient and balanced recovery for patients dealing with post-operative swelling or chronic pain.
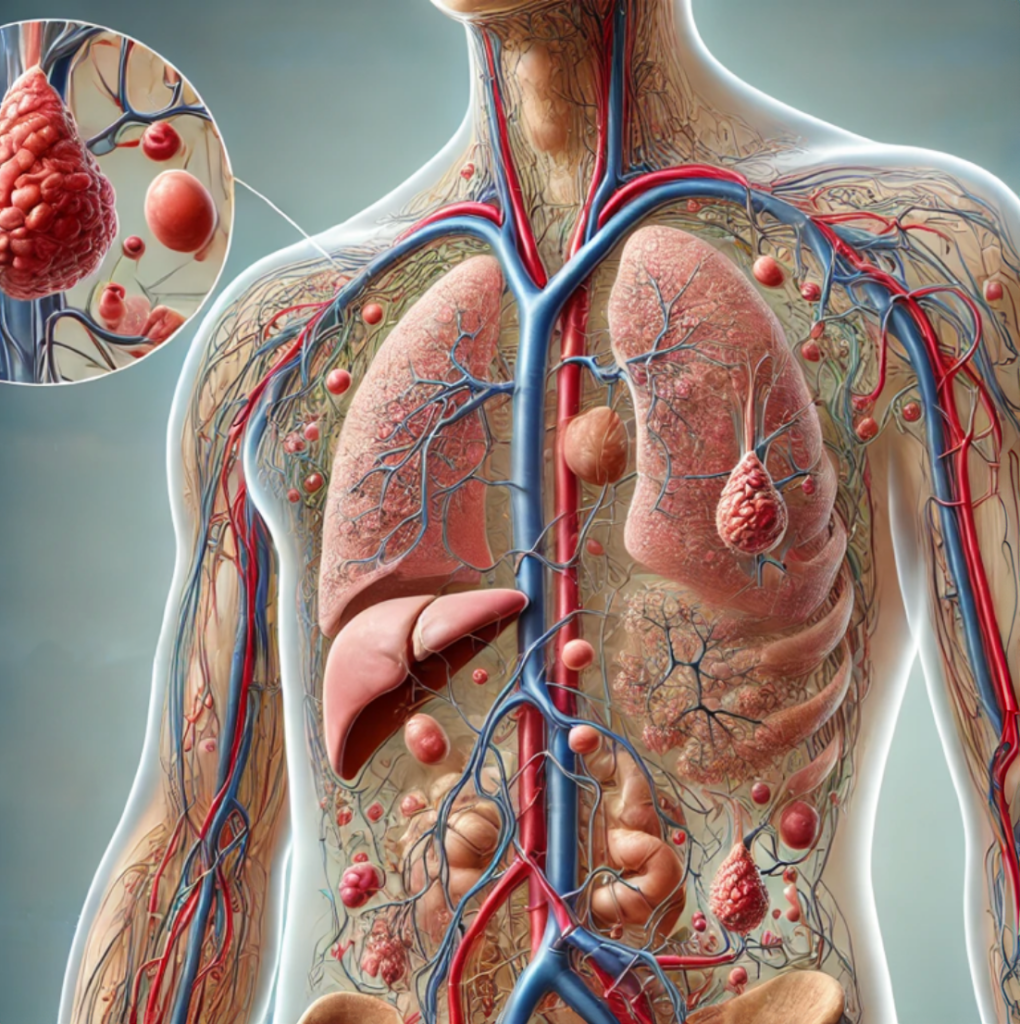
The superficial fascia is a connective tissue layer beneath the skin that supports muscles, nerves, and blood vessels while aiding in fluid movement between tissues. Lymphatic vessels within this layer drain excess fluid, waste, and toxins, helping reduce inflammation and swelling.
An Unhealthy Lymphatic Circulatory System
When the lymphatic system becomes congested or compromised—due to surgery, injury, poor lifestyle habits, or underlying conditions like lymphedema—it can lead to a host of problems.

Lymphatic Congestion Can Cause:
- Swelling and Edema
- Chronic Pain
- Weakened Immune Function
- Tissue Scarring

Tight Fascia Creates Pain
A healthy superficial fascia is essential for mobility, circulation, and overall well-being, as restrictions can
lead to pain and stiffness.
Convenient At-home USE:
With the FDA-cleared over-the-counter (OTC) designation, the Neuroglide is perfect for at-home therapy for people dealing with pain and circulatory issues.
And Neuroglide is simple to use. Just place the Back/Neck Pad on a firm surface such as a couch or therapy table. Position yourself on the pad. The pad will self-adjust to your body structure.

Convenient At-home USE:
With the FDA-cleared over-the-counter (OTC) designation, the Neuroglide is perfect for at-home therapy for people dealing with pain and circulatory issues.
And Neuroglide is simple to use. Just place the Back/Neck Pad on a firm surface such as a couch or therapy table. Position yourself on the pad. The pad will self-adjust to your body structure.
Therapists Trust Us,
Patients Love Us!

PIONEERING NEUROGLIDE CLINICAL STUDIES:

Near-Infrared Fluorescence Imaging (NIRFLI)
Brown Foundation Institute of Molecular Medicine – Houston, Near-Infrared Fluorescence Imaging study (NIRFLI).
Case Report: The effect of automated manual lymphatic drainage therapy on lymphatic contractility in 4 distinct cases. Frontiers in Medicine (Frontiersin.org)
Significant Results:
- Significant lymphatic vessel contractility
- Increased lymphatic flow in torso and limbs
- Reduce Pain
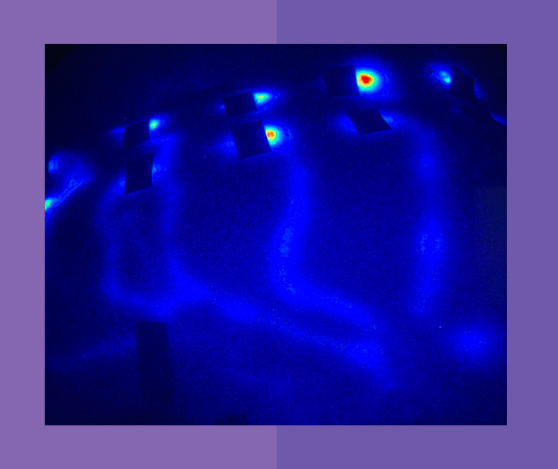
Side view of the back after Neuroglide use.
The Science
NIRFLI of the back to visualize posterior dermal lymphatic flow. Lymph flowed laterally from the spine to the axillary lymph nodes.

Near-Infrared Fluorescence Lymphatic Image of the back.

50 Post-Op Breast Cancer Pain Study
In recent study with fifty post-op breast cancer patients, therapists
performed mastectomy scar tissue therapy with patients using the
Neuroglide at the same time. This study is expected to be published
in 2025.
After just 30-minutes, patients reported significant pain relief, improved relaxation and better sleep. As a result, this clinic adopted the Neuroglide as a new standard of care and branded a new breast health protocol.
SIGNIFICANT PAIN RELIEF | INCREASED RELAXATION | IMPROVED LYMPHATIC CIRCULATION
We support Veterans:
At Eva Medtec, we are deeply committed to supporting our Veterans by providing cutting-edge medical devices that improve their quality of life.
Our Neuroglide Pain Relief and Recovery System is being used to help Veterans manage pain, enhance mobility, and promote faster recovery.
We believe in giving back to those who have served by offering innovative solutions that address their unique health challenges, enabling them to live healthier, more comfortable lives.

Management:

Irene A. Waldridge - Founder and Chief Executive Officer
Irene Waldridge is a seasoned entrepreneur and executive with over 27 years of experience in the medical device industry. As the Founder, CEO, and CTO of Eva Medtec, she holds two patents for the FDA-cleared Neuroglide Pain Relief and Recovery System.
Previously, she founded Tactile Medical, where she developed the groundbreaking Flexitouch System for lymphedema and venous insufficiency, holding four patents. Her leadership roles include CEO, Chairwoman, and CTO, showcasing her pioneering spirit and dedication to improving healthcare solutions.
Robert Wieden - President and Director
Mr. Wieden possesses over 30 years of executive management experience in leading startup or spin-out technology companies and executing commercialization plans, with a direct focus on new business enterprises, leveraging new product/service innovations and bringing them to market.
These past business ventures have led to market experience in conducting business in a number of international markets which has enabled him to build a formidable network of business contacts and strategic partners to augment market opportunities.


Toni M. Hastings - VP of Administration / Operations
With 17 years of experience in managing pain clinics specializing in multidisciplinary solutions for pain management and rehabilitation, Ms. Hastings possesses a deep understanding of executive treatments for patients dealing with chronic spine and musculoskeletal pain conditions.
Her experience includes vendor and supplier management, the establishment of FDA quality control procedures, and the operation of an FDA registered manufacturing facility. Overall, Ms. Hastings extensive background in pain management and medical device development makes her a valuable asset to any organization in these domains.
Jules Fisher - Chief Financial Officer
Jules Fisher’s career is indeed impressive, marked by significant achievements and a broad range of experience across the medical device and pharmaceutical industries. His roles as CFO and his involvement in the successful sale of multiple companies demonstrate his deep expertise in finance, business development, and global operations. His time at Medrad Inc., Possis Medical, and various early-stage companies adds considerable depth to his professional background.
Mr. Fisher’s blend of experience in large-scale operations and early-stage ventures could be highly valuable. His strong academic foundation, including his BS in Accounting, MBA in Finance, and CMA certification, further underscores his capability in financial management and strategic planning.

Advisors:

Mark Melin, MD - Medical Director
Dr. Mark Melin, MD, FACS, RPVI, FACCWS, is a Senior Associate Consultant at the Wound Clinic, Gonda Vascular Center, Mayo Clinic. He specializes in lymphedema management, endothelial glycocalyx research, and the use of micronutrients in wound care. His expertise includes diosmin MPFF, polygenic abnormalities in wound healing, biofilm management, and emerging wound care technologies.
Dr. Melin’s work advances patient care and research in vascular health and wound care. He keeps up with and contributes to developments in emerging technologies within the field of wound care.
Joy Frestedt, PhD. - Chief Scientific Advisor
Dr. Frestedt is also President/CEO for Frestedt Incorporated, Alimentix and the Frestedt Learning Center. She has 40+ years of experience in clinical research, regulatory negotiation, quality system development and business leadership. Frestedt Incorporated and Alimentix are service providers with over 70 experts working in broad CRQE areas of clinical research, regulatory compliance, corporate quality system development
and biomedical engineering.
Dr. Frestedt has experience running clinical trials, conducting laboratory analyses and assisting firms with strategic decisions involving clinical research programs, regulatory strategies and quality system management to compete globally. Dr. Frestedt and her team are well known for their advisory board, clinical, regulatory, quality and training functions.


Frank Aviles - Technical Advisor
Frank Aviles, PT, CWS, FACCWS, CLT-LANA, ALM, AWCC, MAPWCA is the Director of Lymphatic and Wound Healing Services at Hyperbaric Physicians of Georgia. He specializes in treating ulcers, infections, and surgical wounds with advanced therapies. Frank is also President of The Save A Leg, Save A Life Foundation and serves on the board of the Lighthouse Lymphedema Network.
Additionally, he is the Wound Care Clinical Coordinator at Natchitoches Regional Medical Center and owner of Cane River Therapy Services, continuing to impact wound care and therapy.

Tara Newberry - Technical Advisor
Tara Newberry, COTA/L, CLT, OCC is an inventor and Founder of LymphaVibe has 25 years in Occupational Therapy, her focus is on innovation, teamwork, and patient care. Throughout her journey, she successfully merged clinical expertise with business acumen to lead and grow impactful healthcare programs. As the architect behind expanding lymphedema and oncology rehabilitation programs, she has spearheaded large-scale initiatives and developed clinical pathways that have significantly elevated patient care. She has spoken nationally on oncology rehab, artificial intelligence, and medical devices in cancer treatment.
Her work includes research, development, and a patent-pending device for treating swelling and inflammation. Mentoring the next generation of oncology rehab therapists remains a deeply rewarding part of her career.

Doug Goldstein - Clinical Advisor
Dr. Goldstein’s combination of education, board certification, fellowship training, and specialization in performance enhancement make him a
valuable resource for athletes and individuals seeking to optimize their
physical abilities. His work with professional athletes and innovative use of AI technology further showcase his expertise in the field of physical therapy and sports performance.
Dr. Doug provides advisory services to athletes from a wide range of sports,
including the NFL (National Football League), NBA (National Basketball
Association), USA Men’s and Women’s Soccer, MLS (Major League Soccer),
MLB (Major League Baseball), UFC (Ultimate Fighting Championship), and
USA Track and Field. This demonstrates his extensive experience in working
with elite athletes.
Dr. Goldstein earned his Doctorate in Physical Therapy from The University
of Colorado School of Medicine – Physical Therapy Program. This
educational background reflects his strong foundation in physical therapy.
Additionally, he has completed fellowship training, which is an advanced and specialized form of postgraduate education in the field of physical therapy.
He is also board-certified in orthopedics, indicating his expertise in the evaluation and treatment of musculoskeletal conditions.
He is the founder of Launchpad Fitness, an organization that utilizes AI
(Artificial Intelligence) computer vision technology to offer performance
training to youth athletes. This innovative approach enhances training
programs for young athletes.
Use of Proceeds:
Based on the maximum amount of $618,000 is raised.
2%
$12,360
IT / Software
2%
$12,360
Equipment
2%
$12,360
Legal Fees
3%
$18,540
Regulatory
3%
$18,540
Intermediary Fees
3%
$18,540
Travel
4%
$24,720
Liability Insurance
6%
$37,080
Research & Development
8%
$49,400
Office / Warehouse Lease
15%
$92,700
Marketing
22%
$135,960
Mfg. Mat. / Supplies
30%
$188,400
Personnel / Overhead
Partners:
Broker Dealer
Andes Capital
Transfer Agent
KoreTransfer USA LLC
Escrow Facilitator
North Capital Private Securities Corporation
Issuance Technology
KoreConX Inc.
Updates:
Join the Discussion:
Subscribe
Login
Please login to comment
0 Comments
Inline Feedbacks
View all comments

Regulation CF FAQ:
Anyone can invest in a Regulation Crowdfunding offering. Because of the risks involved with this type of investing, however, you may be limited in how much you can invest during any 12-month period in these transactions. If you are an accredited investor (see definition below), then there are no limits on how much you can invest. For Non-Accredited Investors (most fall into this category) the limitation on how much you can invest depends on your net worth and annual income. If either your annual income or your net worth is less than $124,000, then during any 12-month period, you can invest up to the greater of either $2,500 or 5% of the greater of your annual income or net worth. If both your annual income and your net worth are equal to or more than $124,000, then during any 12-month period, you can invest up to 10% of annual income or net worth, whichever is greater, but not to exceed $124,000.
An accredited investor, in the context of a natural person, includes anyone who:earned income that exceeded $200,000 (or $300,000 together with a spouse or spousal equivalent) in each of the prior two years, and reasonably expects the same for the current year, OR has a net worth over $1 million, either alone or together with a spouse or spousal equivalent (excluding the value of the person’s primary residence), OR holds in good standing a Series 7, 65 or 82 license.
After you review the offering statement and information and decide you’d like to invest and how much, you complete the application with the requested information and electronically sign the documentation.
Andes Capital, the Broker Dealer, reviews the information for Anti-Money Laundering and Know Your Customer type reviews
– If you pass the review, Andes Capital initiates the funds via ACH or Credit Card (or you send the wire or check if applicable)
– If you don’t pass the review, Andes Capital or the Issuer will reach out to you to update information to clear you for the reviews or otherwise
Once the funds have been cleared by the escrow agent (funds go directly there), Andes Capital will match your funds with your cleared application for investment, and issue you the stock by validating the subscription agreement and notifying the Transfer Agent to record your ownership on the Issuers capitalization table.
The timeline is generally 2 to 4 weeks but can always happen sooner. It all depends on the information you provide and if there are issues, how quickly we hear back from you.
If we bump up against any “hits” on our reviews, we are required to clear each one of those potential conflicts to evidence there are no issues.
If the information provided is not correct or is incomplete, we will need to reach out and get this corrected.
Even though funds are initiated or you see them pending in your funding source, this does not mean they are in the escrow account for the offering. Based on the payment rails available in the US, these funds may not appear in escrow for 1-2 days and then they have to sit there for a few days until the escrow agent is satisfied there doesn’t appear to be any issues
Matching of payments to approved applications happens in batches, so while all ready to be closed, your application may be in the next batch.
If we bump up against any “hits” on our reviews, we are required to clear each one of those potential conflicts to evidence there are no issues.
If the information provided is not correct or is incomplete, we will need to reach out and get this corrected.
Even though funds are initiated or you see them pending in your funding source, this does not mean they are in the escrow account for the offering. Based on the payment rails available in the US, these funds may not appear in escrow for 1-2 days and then they have to sit there for a few days until the escrow agent is satisfied there doesn’t appear to be any issues
Matching of payments to approved applications happens in batches, so while all ready to be closed, your application may be in the next batch.
Andes Capital Group is the FINRA/SEC registered Broker Dealer who has been engaged by the Issuer to act as the Onboarding Agent for this offering.
Andes Capital Group is NOT soliciting this investment nor making any recommendations by collecting, reviewing and processing your application for investment.
Andes Capital Group conducts Anti-Money Laundering, Identity and Bad Actor Disqualification reviews of the Issuer, and ensures they are a registered business in good standing.
Andes Capital Group is NOT validating or approving the information provided by the Issuer or the Issuer itself.
Contact information is provided for applicants to make inquiries and requests of Andes Capital Group regarding the general application process, the status of the application or general Reg CF regulation related information. Andes Capital Group may direct applicants to specific sections of the Offering Statement to locate information or answers to their inquiry but does not opine or provide guidance on Issuer related matters.
Andes Capital Group is compensated by the Issuer for providing its Broker Dealer Onboarding Agent services as disclosed in the Offering Statement. Andes Capital Group does NOT charge the applying investor any fees whatsoever.
By investing in an Issuer’s Reg CF offering where Andes Capital Group is the Broker Dealer Onboarding Agent, Andes Capital must ensure that you as the investor, do not breach the SEC’s limits on investing in Reg CF Offerings within a 12 month period. While it’s not a full-fledged brokerage account where we custody your holdings or recommend any investments, you will have an account at Andes Capital to track the investments made where Andes Capital was engaged as the Broker Dealer Onboarding Agent.
There is NO cost, charge or no annual fees etc. for this account whatsoever.
There is NO cost, charge or no annual fees etc. for this account whatsoever.
As you are buying a security regulated by the SEC, and as a Broker Dealer we are required by SEC regulations to reasonably ensure Anti-Money Laundering and Know Your Customer reviews are satisfied and that Permitted Investor Limits are not breached, we collect this information to perform the required reviews. Andes Capital Group may share certain details of an applying/completed investor as listed on the subscription agreement, with the Transfer Agent and Escrow Agent if requested for valid purposes of processing an investment application.
Applying investors with questions about the company or its product or the offering should submit their questions in the Discussion section of the investment website. This is a public forum where valid questions will be displayed for all to view, with responses clearly tagged with who the response is from (i.e., the Issuer, Andes Capital Group).
Any questions about the application, how to navigate it, what’s the process, etc. should be directed to Andes Capital Group using the contact information provided at the bottom of the investment website.
